We are proud to share that RSP Systems has successfully closed its Series C investment round at its hard cap of EUR 17 million. This capital injection will continue to support RSP’s fast-paced growth to delivering the world’s first non-invasive wearable glucose monitor.
The financing round is the latest in a series of successful capital raises, bringing the total investment in RSP Systems to over EUR 40 million from a combination of corporate venture and family offices. The company has also received substantial support form the EU Horizon 2020 program.
With this solid financial foundation, we are positioned to complete the transition of our Raman-based technology from its existing portable form factor into a wrist-worn continuous glucose monitor with accuracy rivalling current market leading CGMs.
Anders Weber, RSP Chief Executive Officer, says: “RSP Systems’ in-house expertise in engineering and machine learning, combined with strategic collaborations with industry leaders such as Trumpf Photonics and Ibsen Photonics have allowed us to drive this progress swiftly, and we are excited to be building further successful alliances to complement these over the next quarters.”
Anders Weber, also emphasised the industry’s primary challenge in developing non-invasive glucose monitoring solutions:
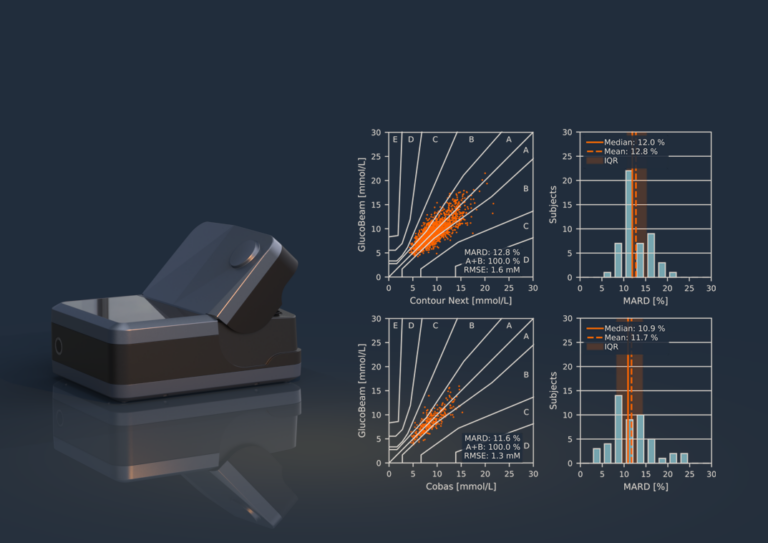
“To date, the biggest challenge the industry has faced in developing a non-invasive glucose monitoring solution is calibration and accuracy, and at RSP Systems we have overcome these key obstacles by not only realising device stability and insensitivity to physiological interferences (including endogenous and exogenous substances) but also by achieving, and maintaining, the required accuracy for practical application.” See our publications here.
The Chairman of the Board of Directors of RSP Systems, Georg von Werz, also expressed strong confidence in the company’s technology, citing the success of its predicate device.
Georg von Werz, says: “The extensive third party clinical trials we conducted with over 160 of our first-generation, portable instruments provided the unequivocal proof that our GlucoBeam device is effective in home use. Since then we have conducted numerous further successful trials both in-clinic and out-patient and continue to document this through peer-reviewed papers. In this we are alone in the field.”
RSP will be attending the 84th ADA Scientific Sessions in Orlando, June 21-24, 2024.