Remember to Celebrate – and enjoy the journey
This week we were visited by an assessor who executed our first ISO1345:2016 surveillance audit.
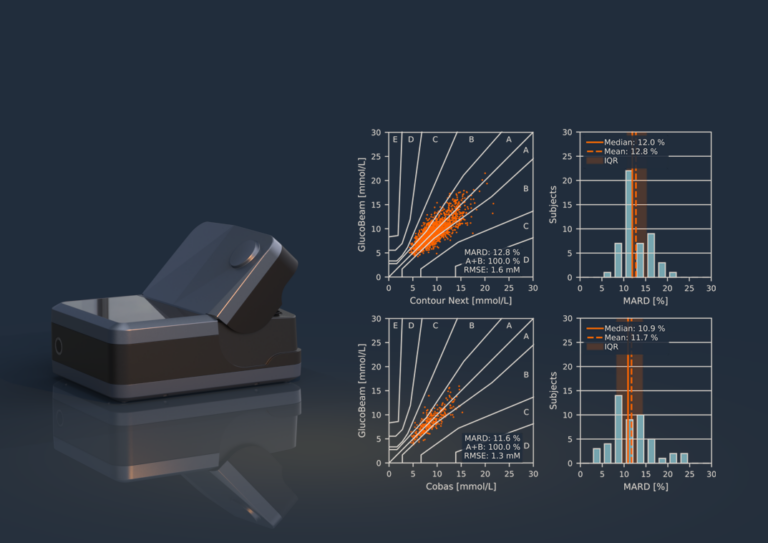
The purpose of an ISO 13485:2016 surveillance audit is to ensure that a company has procedures in place to ensure that we actually do what we say with respect to the development of a medical device. The goal is to ensure the safety and reliability of the medical device.
The visit did not reveal any non-conformities in our quality management system.
We are very proud because this proves that as a team, we are continually working hard to ensure that we develop a safe and reliable medical device for people with diabetes.